- Home
- Forums
- Skin diseases Forum
- Living with skin conditions
- Necrotizing fasciitis (flesh-eating disease)
Patients Skin diseases
Necrotizing fasciitis (flesh-eating disease)
- 15 views
- 0 support
- 1 comment
All comments

Unregistered member
20/09/2015 at 21:52
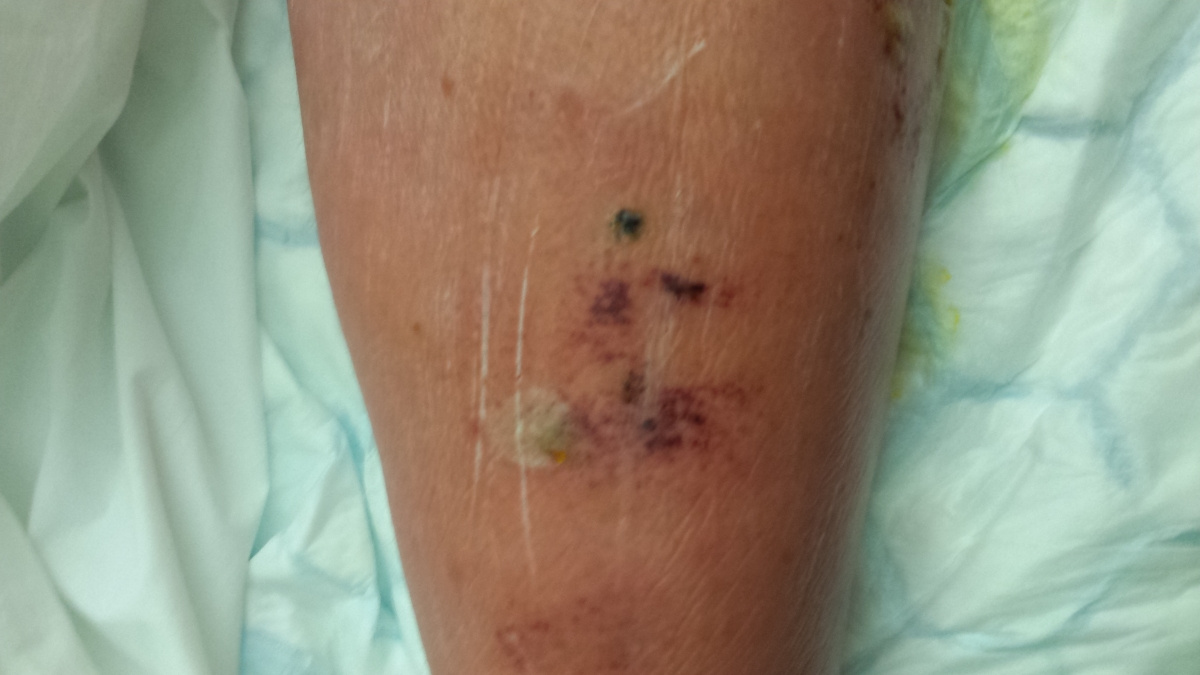
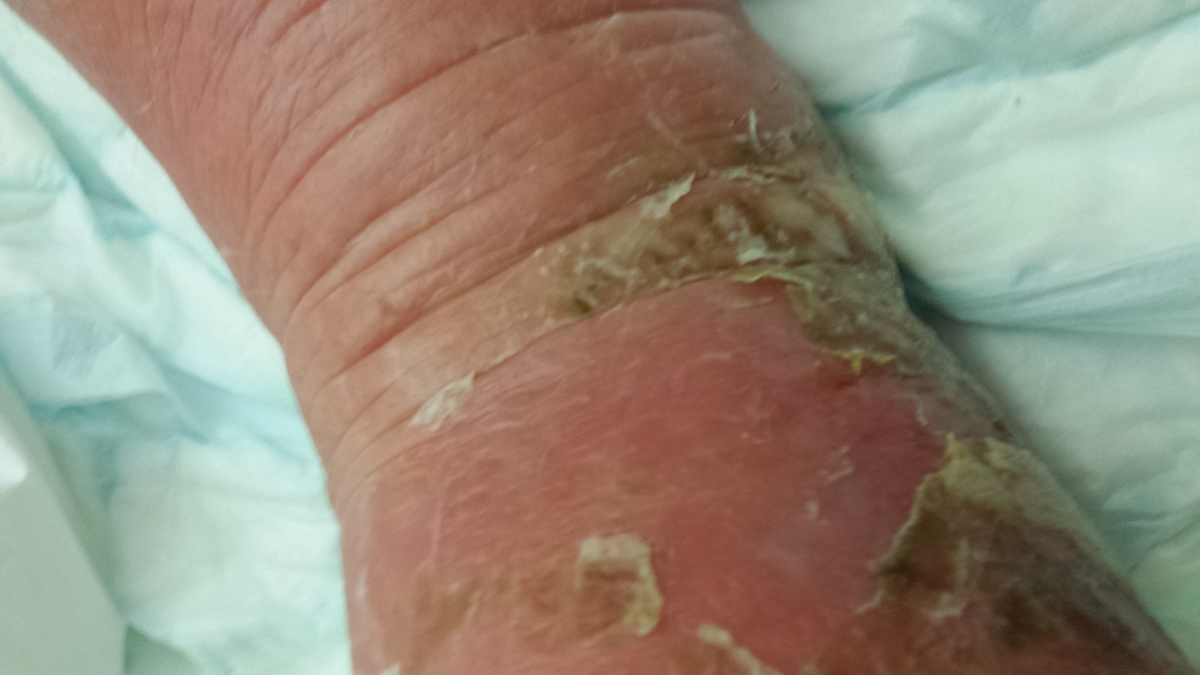
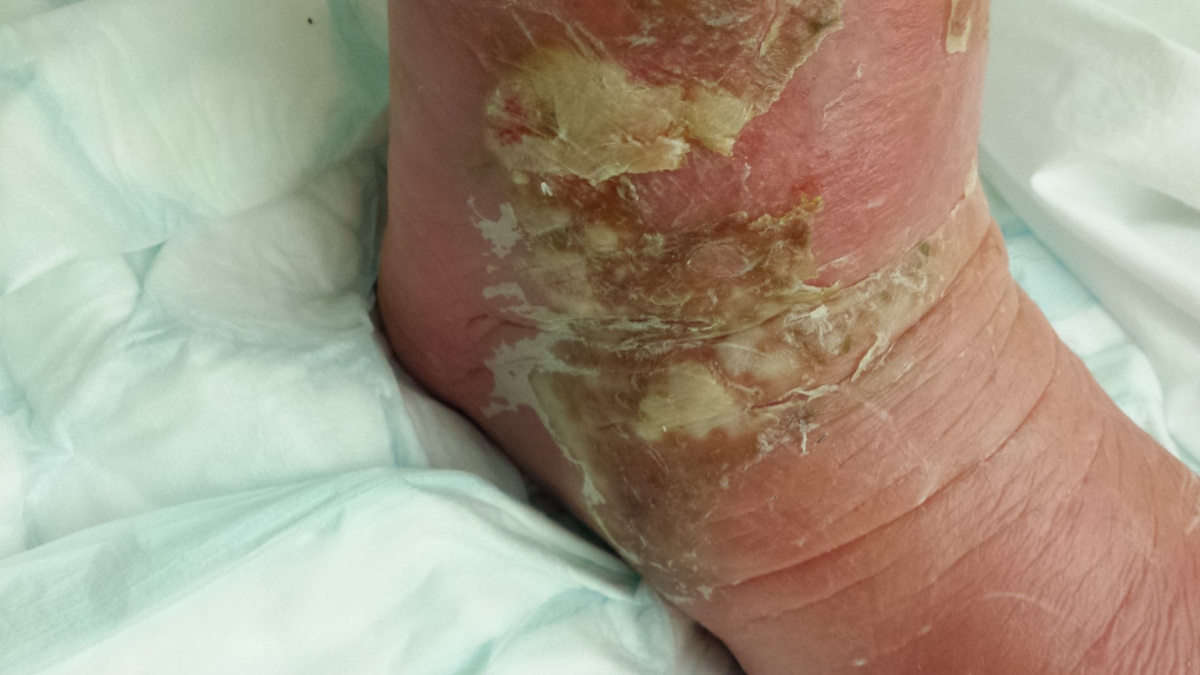
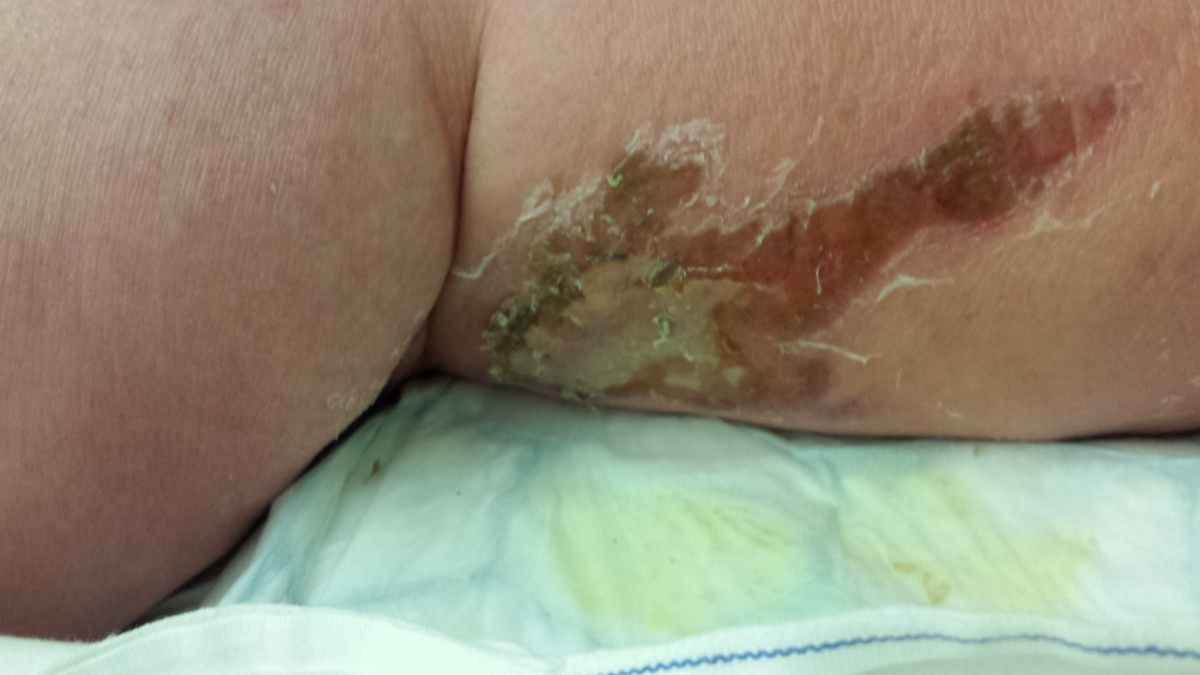
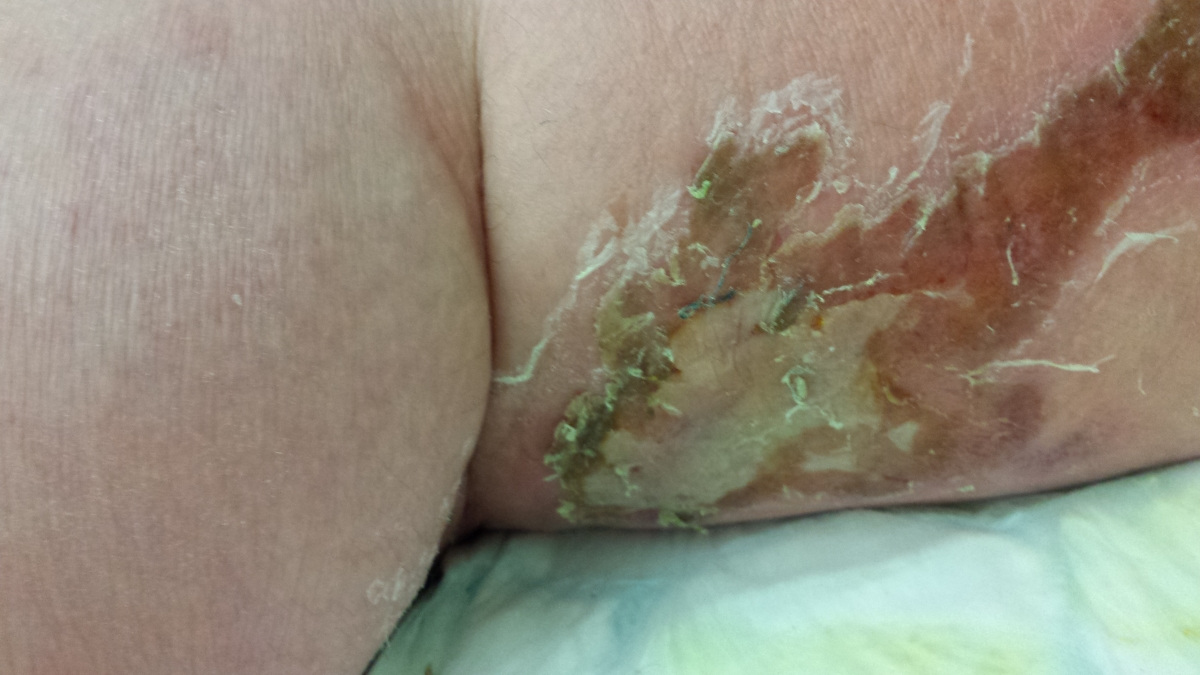
Here are the photos - NOT for the squeamish!!! The first one shows the site of the bite, after it turned black.
Give your opinion
Members are also commenting on...
Articles to discover...
Subscribe
You wish to be notified of new comments
Your subscription has been taken into account









Unregistered member
Necrotizing fasciitis (flesh-eating disease)
In early March 2015, my 38 year old son was bitten by an insect while walking his dog. He took no real notice of the bite, and it is only subsequently that we have realised this is what caused him to develop Necrotizing Fasciitis.
The first thing we knew, was when he started to get a swollen leg. It was thought to be cellulitis. It continued to deteriorate over the next few days, during which he developed flu like symptoms. As my step-daughter and family had recently had a flu-like illness and he had called at the house during it, we just thought he had caught it. When he started to projectile vomit and had an extremely high temperature, we decided we better take action. We took him to casualty and he was admitted.
They did not know what was causing the problem and put the swelling down to some sort of fluid retention problem. He had kidney tests and his kidney function was failing, so he was transferred to a hospital in a nearby city who had a specialist in urology.
We really thought he would need dialysis but they managed to stabilise the deterioration and transfer him back to our local hospital.
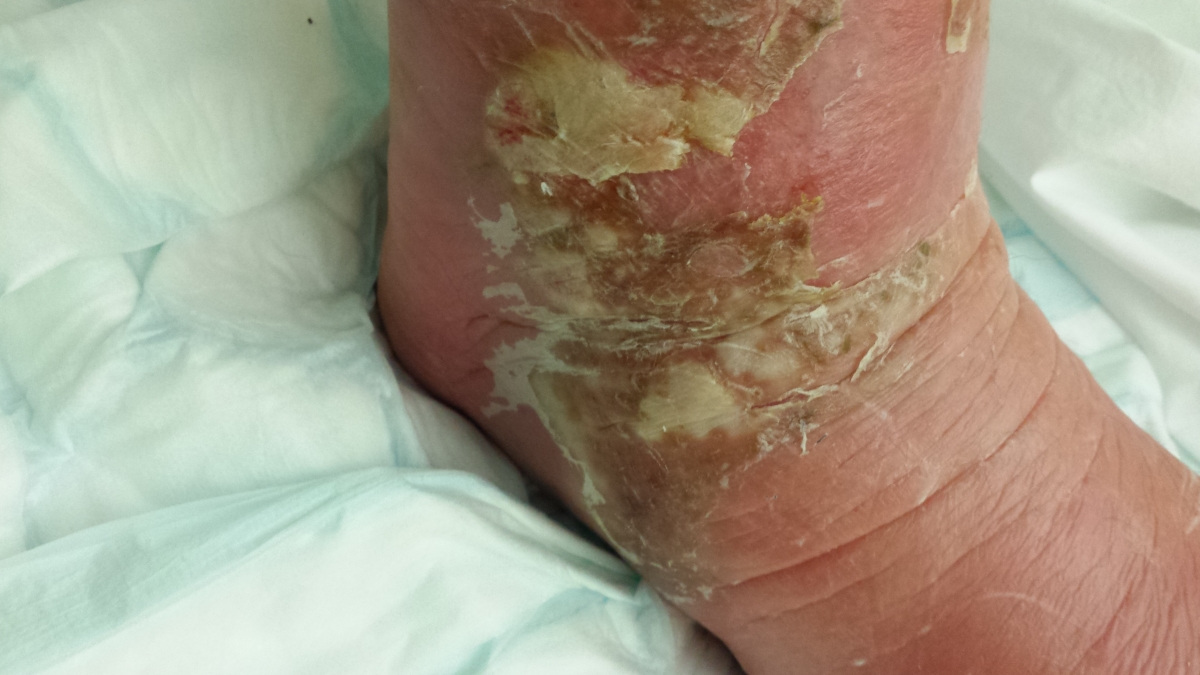
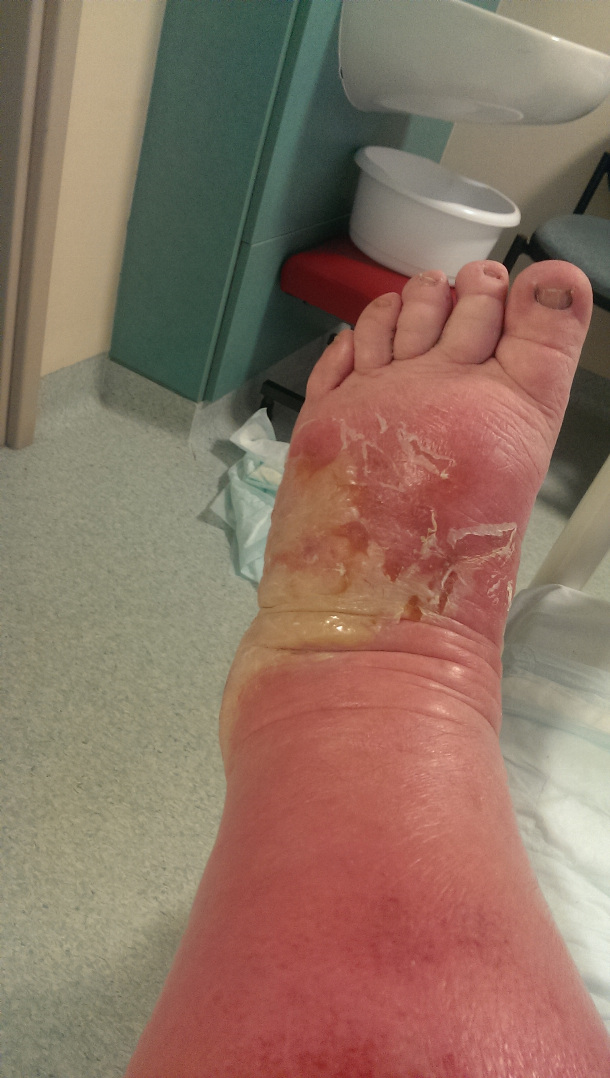
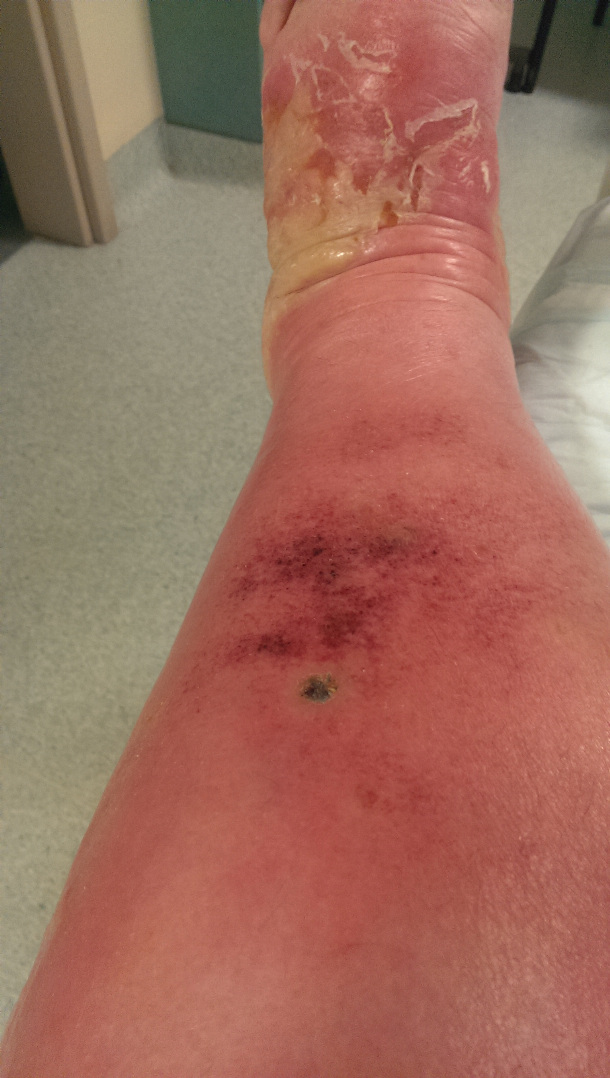
By now, his swollen leg had started to develop wounds, which were oozing puss. His leg was basically splitting open in various places and the insect bite had become, large, risen and black. He was delirious, paranoid and delusional with the infection.
I attach a couple of pictures of it – not for the squeamish!!!
To cut a long story short, it transpired that he had developed Necrotizing Fasciitis, a flesh eating disease and had Sepsis too. His vital organs were under attack and we first thought we would lose him completely, then that he would lose the leg.
Fortunately he survived, kept the leg and is now home. He still has many problems, the leg still swells to over three times the size of the other, he needs to have some treatment on his lymph glands and is having regular heart and kidney tests. He is still one very poorly bunny and probably will be for some time – apparently we are talking years, not months.
If you have never heard of this condition, here are some of the facts about it. My research sources for this were Centres for Disease Control and Prevention website, http://www.cdc.gov/features/necrotizingfasciitis/ and MedicineNet.com at http://www.medicinenet.com/necrotizing_fasciitis/article.htm.
Basic Facts
Necrotizing fasciitis (neck-ro-tie-zing Fas-e-i-tis) refers to a rapidly spreading infection, usually located in fascial planes of connective tissue that results in tissue death (necrosis).
Different types of bacterial infection can cause necrotizing fasciitis.
The majority of cases begin with an existing infection, most frequently on an extremity or in a wound.
Necrotizing fasciitis is a serious condition that is often associated with sepsis and widespread organ failure.
Treatment of an infection caused by flesh-eating bacteria involves rapid antibiotic administration and/or surgical debridement of the wound areas as well as supportive measures such as insertion of a breathing tube, intravenous administration of fluids, and drugs to support the cardiovascular system.
Good hygiene and wound care can reduce the chance of developing the disease; necrotizing fasciitis is not usually contagious but it is possible to transmit infectious agents to other people (cross-contaminations of wounds, for example).
Immunosuppressed individuals (for example, diabetics, elderly, infants, those with liver disease, alcoholics, or those taking immunosuppressive drugs such as chemotherapy for cancer) are at higher risk to develop the disease.
The prognosis depends on how fast the infection is diagnosed and treated and the patient’s response to treatments; outcomes usually range from fair to poor with complications including tissue loss and or amputation of limbs.
What is necrotizing fasciitis?
Necrotizing fasciitis is a serious bacterial skin infection that spreads quickly and kills the body's soft tissue. (Necrotizing means "causing the death of tissues.") Accurate diagnosis, prompt treatment with antibiotics through a vein, and surgery are important to stopping this infection that can become life-threatening in a very short amount of time.
Do different types of necrotizing fasciitis exist?
Variations of necrotizing fasciitis are placed by some investigators into three general groups or types, roughly based on the type of organisms causing the infection and some clinical findings that vary from patient to patient.
Type 1 is either caused by more than two bacterial genera (polymicrobial) or by the infrequently found single bacterial genus such as Vibrio or fungal genera such as Candida.
Type 2 is caused by Streptococcus bacteria.
Type 3 is caused by Clostridium bacteria.
What causes necrotizing fasciitis?
Bacteria cause most cases of necrotizing fasciitis; only rarely do other organisms such as fungi cause this disease. Other organisms may rarely cause necrotizing fasciitis, but when they do, the resulting infections are often difficult to treat successfully.
In general, the bacteria that cause necrotizing fasciitis utilize similar methods to cause and advance the disease. Most produce toxins that inhibit the immune response, damage or kill tissue, produce tissue hypoxia, specifically dissolve connective tissue, or do all of the above. In polymicrobic infections, one bacterial genus may produce one toxic factor while different types of bacteria may produce other toxins that disintegrate damaged tissue cells or connective tissue. In general, this disease is not contagious, but the organisms that may lead to its development are contagious, usually by direct contact between people or items that can transfer the bacteria.
Most cases of necrotizing fasciitis occur randomly and are not linked to similar infections in others. The most common way of getting necrotizing fasciitis is when the bacteria enter the body through a break in the skin, like a cut, scrape, burn, insect bite, or puncture wound.
Most people who get necrotizing fasciitis have other health problems that may lower their body's ability to fight infection. Some of these conditions include diabetes, kidney disease, cancer, or other chronic health conditions that weaken the body's immune system. If you're healthy, have a strong immune system, and practice good hygiene and proper wound care, your chances of getting necrotizing fasciitis are extremely low.
How is necrotizing fasciitis diagnosed?
Often a preliminary diagnosis of necrotizing fasciitis is based on the patient's symptoms, including the medical and exposure history as described above. Organisms isolated from necrotizing fasciitis need to have studies done to determine antibiotic resistance, because many organisms causing the disease are multidrug resistant. A surgeon needs to be consulted early to help obtain tissue samples and to be involved with potential treatment protocols. Although X-rays occasionally show gas in tissues, investigators suggest doing Doppler ultrasound, CT, or MRI studies to help show gas in tissues and to help delineate the extent of the infection. Most physicians run additional tests such as white blood cell (WBC) counts (elevated in necrotizing fasciitis), BUN (blood urea nitrogen), sodium (both decreased in necrotizing fasciitis), and other tests to monitor the patient.
What are necrotizing fasciitis symptoms and signs?
The symptoms often start within hours after an injury and may seem like another illness or injury. The majority of infected individuals who develop necrotizing fasciitis begin with an existing infection (cellulitis, abscess, or wound), most frequently on an extremity or in a wound or surgical site.
The initial infection can be from almost any cause, for example, cuts on the skin, puncture wounds, surgical incisions, or rarely, insect bites. Instead of healing, the infected site can show erythema (redness) and swelling. The site may be very sensitive to pain, even past the area of erythema and may tingle. Some people complain of pain or soreness, similar to that of a "pulled muscle." The skin may be warm with red or purplish areas of swelling that spread rapidly. There may be ulcers, blisters or black spots on the skin. Patients often describe their pain as severe and way out of proportion to how the painful area looks when examined by a doctor. Fever, chills, fatigue (tiredness) or vomiting may follow the initial wound or soreness. These confusing symptoms may delay a person from seeking medical attention.
Although early symptoms resemble those of cellulitis, progressive skin changes such as skin ulceration, thin-walled fluid-filled blisters (bullae) formation, necrotic scars (black scabs), gas formation in the tissues, and fluid draining from the site can occur rapidly as the infection progresses.
If you think you may have these symptoms after a wound, see a doctor right away.
Some patients can become septic (meaning the infection has spread to the bloodstream and throughout the body) before the skin changes are recognized, especially when flesh-eating disease begins in deep facial planes.
Type 1 often occurs after trauma or surgery and may form little or undetectable amounts of gas. Type 2 usually occurs after more simple skin trauma (cuts, abrasions, and insect bites) and infects more superficial facial planes with almost no gas formation. Type 3 usually occurs after trauma or after wounds become contaminated with dirt that contains Clostridium bacteria, which produce gas in tissues (gangrene) and necrotic eschars. However, symptoms for types 1-3 are not definitive, and symptoms vary widely which is why some investigators prefer to define individual patients' disease by the organism(s) isolated from the patient rather than assigning a type label.
Prompt Treatment Needed for Necrotizing Fasciitis
The first line of defense against this disease is strong antibiotics given through a needle into a vein. But because the bacterial toxins can destroy soft tissue and reduce blood flow, antibiotics may not reach all of the infected and dying areas. This is why rapid surgical exploration and removal of dead tissue—in addition to antibiotics—is often critical to stopping the infection.
At the time of preliminary diagnosis, the patient needs to be hospitalized and started on intravenous antibiotics immediately. The initial choice of antibiotics can be made based upon the types of flesh-eating bacteria suspected of causing the infection, but many doctors believe that multiple antibiotics should be used at the same time to protect the patient from methicillin-resistant Staphylococcus aureus (MRSA), as well as infections with anaerobic bacteria, and polymicrobic infections. Antibiotic susceptibility studies, done in the laboratory after the infecting organism(s) has been isolated from the patient, can help the physician choose the best antibiotics to treat the infected individual.
A surgeon needs to be consulted immediately if necrotizing fasciitis is suspected or preliminarily diagnosed. Debridement (removal) of necrotic (dead) tissue and collection of tissue samples, needed for culture to identify the infecting organism, are done by a surgeon. The type of surgeon consulted may depend on the area of the body affected. As is the case for immediate antimicrobial therapy, early surgical treatment of most cases of necrotizing fasciitis can reduce morbidity and mortality.
Many patients with necrotizing fasciitis are very sick and most require admission to an intensive-care unit. Sepsis and organ failure (renal, pulmonary, and cardiovascular systems) need to be treated aggressively to increase the patient's chance for recovery. Treatments such as insertion of a breathing tube, intravenous administration of fluids, and drugs to support the cardiovascular system may be required. Although not available in many hospitals, hyperbaric oxygen therapy (oxygen given under pressure with the patient in a specialized chamber) is sometimes used in treatment as the oxygen can inhibit or stop anaerobic bacterial growth and promote tissue recovery. This therapy does not replace antibiotics or surgical treatment. However, hyperbaric oxygen therapy has been shown by researchers to further reduce morbidity and mortality by about 10%-20% in some patients when used in conjunction with antibiotics and surgery
How is necrotizing fasciitis prevented? Is necrotizing fasciitis contagious?
Necrotizing fasciitis does not begin unless an infection has already started in tissue; immediate effective treatment of any infection is likely to prevent the disease. Further, anything that can help prevent infections will help prevent necrotizing fasciitis. Practices such as hand washing, checking extremities for cuts or wounds if you have diabetes, avoiding physical contact with people who carry MRSA, and good hygiene practices help prevent initial infections that may lead to flesh-eating disease. Immunosuppressed patients should be very careful not to get infections, and people with liver disease should avoid eating seafood that may be contaminated with Vibrio vulnificus. People with liver disease should not have any infections or cuts in the skin exposed to warm seawater to avoid necrotizing fasciitis caused by Vibrio vulnificus.
Physicians, surgeons, and other caregivers play an important role in prevention. Cases of necrotizing fasciitis may occur when surgical sites become infected. Consequently, physicians need to use sterile techniques when doing surgery and adhere to hospital practices such as glove and gown coverage to help prevent infection spread in hospitalized patients. Careful surgical techniques in sites that can easily become contaminated are required. Some examples of such sites are bowel surgery, episiotomy (surgically enlarging the vaginal outlet), and debridement with closure of traumatic wounds.
Who is at risk to get necrotizing fasciitis?
Theoretically, anyone with an infection has a small risk of getting necrotizing fasciitis; the risk begins to increase if the infection occurs in immunosuppressed individuals (for example, diabetics, elderly, infants, those with liver disease, or those taking immunosuppressive drugs such as chemotherapy for cancer). Visible infections (skin, hair follicles, fingernails, visible trauma sites) are more likely to be noticed and treated than some deep infections. Patients who have any deep infections (muscle, bone, joint, gastrointestinal) are at somewhat higher risk for the disease because the initial infection and subsequent spread is usually not as noticeable as more visible infections.
Necrotizing fasciitis has interesting demographics; more males than females are affected (about three to one), and Vibrio vulnificus infections seem limited to coastal areas with warm water where the organisms are found associated with seafood and contaminated water.
What is the prognosis (outcome) for patients with necrotizing fasciitis? What are complications of necrotizing fasciitis?
Untreated necrotizing fasciitis has a poor prognosis; death or severe morbidity (for example, limb loss) is the frequent outcome.
The worst complication of this disease is rapid advancement that results in death. Other serious complications include tissue loss and organ damage to areas that have to be removed by surgery to limit the disease. Other complications include amputation, sepsis, kidney failure, and extensive scarring.