Castleman disease and HIV: A focused look at the HHV-8 virus
Published 20 Jan 2021 • By Charlotte Avril
Castleman disease is a non-cancerous, non-malignant disease affecting the lymph nodes. Both non-communicable and non-hereditary, there are several distinct forms of the disease in terms of both presentation and care.
As a rare condition with an uncertain cause, many questions remain unanswered to this day and researchers are working to advance the understanding of this disease and its care. If you wish, you can find more detailed information on current research advances in Castleman disease in the article "Castleman Disease - What is the current research?".
What are the links between HHV-8-associated Castleman disease and HIV infection? What are the consequences of being co-infected and how is it treated?
We tell you everything in our article!
Higher risk for HIV-positive patients
Although multicentric Castleman disease (MCD) is rare, the risk of developing the disease is greater in HIV-positive patients. One reason for this is the fact that these individuals have immune systems weakened by HIV infection, and the associated almost universal presence of human herpes virus 8 (HHV-8) in cases of diagnosed Castleman disease (CD). Several studies link HHV-8 to CD and have shown that HHV-8 can also infect immune cells by preventing their normal development. This explains why co-infection with HIV, which itself weakens the immune system, leads to a higher probability of developing MCM and associated diseases.
What is HHV-8 and how is it transmitted?
HHV-8 is a large, double-stranded DNA virus of the Herpesviridae family, from the Gammaherpesvirinae subfamily and rhadinovirus genus. It is the only rhadinovirus known to occur in humans. According to documented statistics, this virus, like HIV, particularly affects men in the homosexual community, and also affects more severely people from countries with a high prevalence of the virus (mainly in Africa).
Although it is not yet fully understood, HHV-8 transmission seems to be mainly transmitted via saliva and sexual intercourse. Indeed, oral to genital transmission is considered particularly infectious as well as transmission by direct contact or via droplets (sneezing, coughing, etc.), also known as horizontal transmission. Lastly, it is believed that there is a risk of transmission during organ transplantation. Given that the rate of seroprevalence of this virus increases with age, this suggests that another means of transmission exists, but it has not yet been identified.
Like all herpesviruses, HHV-8 is inactive in most of the cells it infects and is therefore most often asymptomatic when the immune system is functioning properly. If this is not the case, various complications can occur.
HHV-8-associated syndromes
There are currently three conditions associated with the virus, the most common of which is Kaposi's sarcoma.
Kaposi's sarcoma exists in different forms that can either develop independently of the AIDS virus or, most frequently, in patients co-infected with HIV and HHV-8: this association is observed in up to 75% of cases. A sharp increase in the number of cases of Kaposi's sarcoma was observed following the AIDS epidemic, particularly on the African continent where the HHV-8 virus is endemic. Kaposi's sarcoma (SK) is an angioproliferative disorder of the vascular endothelium which presents itself in the form of skin tumours, also sometimes reaching the lymph nodes and internal organs.
Another very rare syndrome associated with HHV-8 is primary effusion lymphoma (PEL)). It is characterised by effusions of pleural, peritoneal and pericardial fluid in which cancerous cells can be found. This effusion can cause main symptoms: dyspnoea when it occurs in the heart or lungs, and abdominal distension when it is occurs in the peritoneum (the membrane lining the abdominal cavity). The prognosis in PEL is usually rather negative, even more so when the disease is coupled with HIV infection (which represents the majority of cases), due to the deterioration of the immune system, which greatly facilitates the onset and evolution of the disease.
Finally, as mentioned earlier, the HHV 8 virus, which can be found in the lymph nodes or blood, also represents a potential cause of multicentric Castleman disease (MCD). To learn more about CD, its different forms, symptoms and possible treatments, you can read our article "Castleman disease: Everything you need to know!". Studies have shown that the MCD in HIV-positive patients is consistently linked to HHV-8 infection, whereas HHV-8 is observed in only half of the MCD cases without HIV infection. Therefore, it is important to note that HHV-8-associated cases of Castleman disease are more severe, with a more severe prognosis and more frequent systemic symptoms. The rapid evolution and difficulty of diagnosis in some of areas of the world, particularly in Africa, are the reasons why, as with primary effusion lymphoma (PEL), the prevalence of MCD is not well known in these areas of high viral endemicity.
MCD is strongly linked to HIV and is often associated with Kaposi's sarcoma and in a few cases even with PEL, especially in immunocompromised patients. The prognosis of isolated MCD has improved significantly since the introduction of current treatments. However, in the most severe cases, in combination with other syndromes mentioned for example, the prognosis can quickly deteriorate and death often occurs due to high-grade lymphoma and multi-organ failure.
Castleman disease (CD) associated with HHV-8 in the context of HIV infection: Presentation and treatment
In the case of HIV infection, the disease presents itself in a similar way as severe forms of idiopathic multicentric Castleman disease (iMCD). However, complications can occur:
- Haemophagocytic lymphohistiocytosis (HLH):
- Anaemia with signs of haemolysis (decrease and destruction of red blood cells), thrombocytopenia (decrease in platelets) and sometimes pancytopenia (a fatal condition including a decrease in platelets, red blood cells and white blood cells).
- Rapid onset and fatal multi-organ failure is possible.
- Increased autoimmune cytopenia (reduction in the number of mature blood cells)
- Autoimmune haemolytic anaemia (AHA): a blood disorder characterised by an abnormal decrease in the number of red blood cells.
- Immune thrombocytopenic purpura (ITP): a blood disorder characterised by a decrease in the number of platelets in the blood.
- Kaposi sarcoma: its presence often facilitates diagnosis of MCD.
- Distinct skin lesions on the skin or mucous membrane.
- Damage to the respiratory or gastrointestinal tract.
- Oedemas or serous effusions: PEL can be found as mentioned earlier or oedemas along with pleurisy (infection of the pleura in the lungs).
The diagnosis is easily made when there is one of these complications, especially if there are Kaposi lesions, or if the HHV-8 virus is detectable in the blood (using a quantitative PCR test).
When treating CD in the context of HIV infection, attention must be paid to the control of viral replication (plasma viral load) and the severity of the immune deficiency (CD4+ T cell count). It is also necessary to investigate the possibility of clinical Kaposi sarcoma, as this will influence treatment.
The following diagram, from the French High Authority for Health, indicates the treatments to be implement according to the patient's situation:
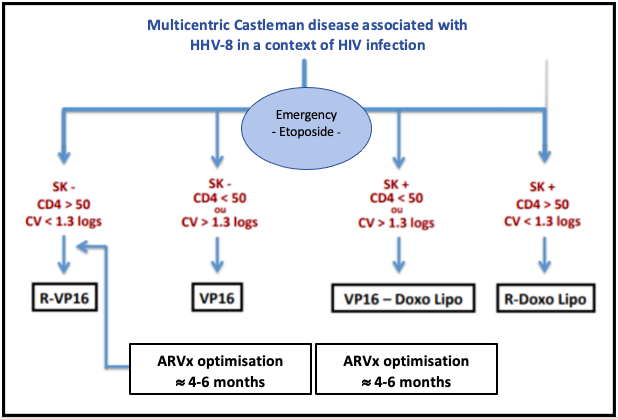
Source: National Diagnosis and Care Protocol with Regards to Castleman Disease, from the French High Authority for Health (2019)
Therefore:
- In the absence of progressive Kaposi sarcoma and in a context of treated and controlled HIV with a CD4 count > 50/mm3, the treatment is based on the R-VP16 (rituximab/etoposide) regimen of 4 weekly cycles (Level 4, category 2A).
- In the absence of progressive Kaposi sarcoma but in a context of uncontrolled HIV or with a CD4 count < 50/mm3, the treatment combines etoposide, which can be used orally on a weekly basis (70 -100 mg/m2) and the introduction or optimisation of antiretroviral treatment (ARVx). Once the viral load is under control and the CD4 count > 100/mm3, the R-VP16 regimen can be offered.
- In a patient with progressive Kaposi sarcoma but with controlled HIV, the treatment will associate rituximab and liposomal doxorubicin (Level 4, category 2B).
- In a patient with progressive Kaposi's sarcoma and uncontrolled HIV or a CD4 count < 50/mm3, a combination of etoposide and liposomal doxorubicin with the introduction or optimisation of antiretroviral therapy (ARVx) is preferred
How can HHV-8-associated Castleman disease be prevented?
There a few simple techniques that can help prevent HHV-8 infection. As the virus is not very resistant to outdoor environments, basic hygiene, such as regular hand washing, can limit the transmission of the virus. On the other hand, given that oral-genital and oral-anal intercourse represent major risks for contamination, the use of a condom or a dental dam is strongly recommended.
Screening for HHV-8 is also an essential tool in the prevention of the disease, particularly to identify asymptomatic carriers of the virus. It should also be stressed that the prevention of CD and its complications requires HIV screening. Antiretroviral treatment may also be necessary in some cases to ensure that the immune system of the affected patients can fight HHV-8.
To go further:
For more information, feel free to visit the CDCN (Castleman Disease Collaborative Network) website.
You can also join the Castleman Disease community on Carenity to share your experience, find support and exchange information with other patients or their relatives.
Sources:
- Sarcome de Kaposi, Institut Pasteur
- Maladie de Castleman multicentrique associée à un sarcome de Kaposi liés au virus HHV-8 : étiologies d’une polyadénopathie chez un patient infecté par le VIH, ScienceDirect
- L’herpèsvirus humain 8 (HHV-8) : aspects cliniques, épidémiologiques et clonalité des maladies tumorales associées, Académie Nationale de Médecine
- Protocole National de Diagnostic et de Soins (PNDS) Maladie de Castleman, Haute Autorité de Santé
- Maladie de Castleman associée au virus HHV-8, Castleman - Centre de référence des maladies rares

 Facebook
Facebook Twitter
Twitter